Resources for MuGard® Oral Mucoadhesive
MuGard has been studied in clinical trials. You may access many of the studies and publications below. Full Prescribing Information, the MuGard prescription form, a patient brochure and an HCP information sheet are also available here.
MuGard® Clinical Studies
Cancer – Interdisciplinary International Journal of the American Cancer Society

Allison RR, et al. Multi-institutional, randomized, double-blind, placebo-controlled trial to assess the efficacy of a mucoadhesive hydrogel (MuGard) in mitigating oral mucositis symptoms in patients being treated with chemoradiation therapy for cancers of the head and neck. Cancer. 2014;120(9):1433-1440.
Oncology Nurse Advisor
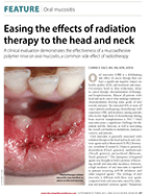
Carrie F. Daly, MS, RN, APN, AOCN. Easing the effects of radiation therapy to the head and neck. Oncology Nurse Advisor, September/October 2013.
Therapeutic Advances in Medical Oncology

Parvin F. Peddi, et al., University of California, Los Angeles. Phase II randomized trial of a non-steroidal mouth wash [MuGard] for prevention and treatment of stomatitis in women with hormone receptor positive breast cancer treated with everolimus.
MuGard®, an Oral Mucoadhesive Hydrogel

MuGard, an oral mucoadhesive hydrogel, reduces the signs and symptoms of oral mucositis in patients with lichen planus: a double-blind, randomized, placebo-controlled pilot study.
Additional Resources
Support services are available through Soleva Assist, including:
- Medical information
- How to obtain product
- Report an adverse event or product complaint
- Reimbursement support
- Patient assistance
For more information, contact Soleva Assist toll-free at 1-877-319-7272
Indication and Important Safety Information
Indication: MuGard® Oral Mucoadhesive is indicated for the management of oral mucositis/stomatitis (that may be caused by radiotherapy and/or chemotherapy) and all types of oral wounds (mouth sores and injuries), including aphthous ulcers/canker sores and traumatic ulcers, such as those caused by oral surgery or ill-fitting dentures or braces.
Contraindications: MuGard is contraindicated in patients with known hypersensitivity to any of the ingredients in the formulation.
Special Precautions for Use: Patients should avoid eating or drinking for at least one hour after using MuGard. After use, patients should replace the bottle cap and tightly seal the bottle. This product should not be used after the expiration date shown on the carton and product label. Do not use this product in patients with known sensitivity to any of the product’s ingredients. Dilution of the product prior to use is not recommended.
You may report a MuGard® adverse event to Soleva Pharma at 877-319-7272 or by emailing to
medinfo@solevapharma.com.