Oral mucositis (OM) is a painful and debilitating side effect of many cancer treatments
Oral mucositis: an inflammation of mucous membranes in the oral cavity (mouth, gums, tongue), which usually appears as red, ulcer-like sores1,2
Oral mucositis is associated with oral pain and swelling, and development of oral mucositis may cause difficulty eating, swallowing, talking and sleeping. Oral mucositis may result in the need for opioids or other analgesics for pain management, lead to weight loss, and create a pathway for infection.
Stomatitis is another word sometimes used to describe inflammation or sores of the mucus membranes inside the mouth.
OM incidence
In patients being treated for cancer, likelihood of experiencing OM varies by tumor type and treatment regimen.
By tumor type

Head and neck cancer receiving radiation therapy3
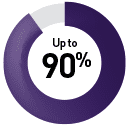
Hematopoietic stem cell transplant4
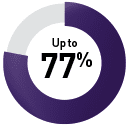

Head and neck cancer receiving chemotherapy5
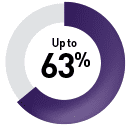
Colorectal cancer5
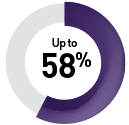
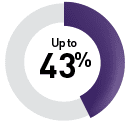
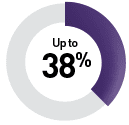
By targeted therapy
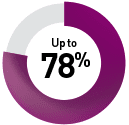
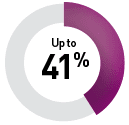
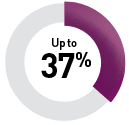
Sunitinib9
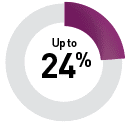
Additional targeted therapeutics associated with risk of OM include bevacizumab, cetuximab, erlotinib, gefitinib, and sorafenib.11
OM continues to be a problem for patients undergoing treatment for cancer.1,12 Management of OM should be considered for patients who face OM associated with treatment of cancer.1,12,13
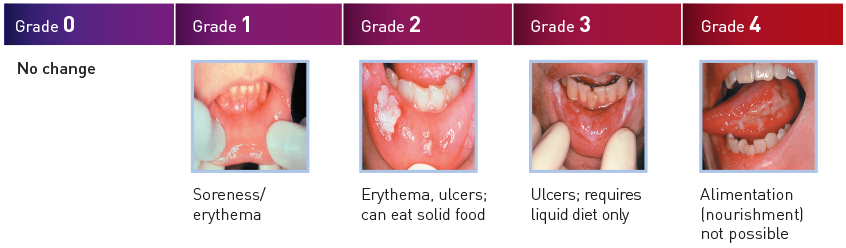
The WHO Oral Toxicity Scale
The WHO (World Health Organization) classification of oral toxicity combines descriptions of mucosal changes, pain, and functionality into a single composite score.1,14,15
Indication and Important Safety Information
Indication: MuGard® Oral Mucoadhesive is indicated for the management of oral mucositis/stomatitis (that may be caused by radiotherapy and/or chemotherapy) and all types of oral wounds (mouth sores and injuries), including aphthous ulcers/canker sores and traumatic ulcers, such as those caused by oral surgery or ill-fitting dentures or braces.
Contraindications: MuGard is contraindicated in patients with known hypersensitivity to any of the ingredients in the formulation.
Special Precautions for Use: Patients should avoid eating or drinking for at least one hour after using MuGard. After use, patients should replace the bottle cap and tightly seal the bottle. This product should not be used after the expiration date shown on the carton and product label. Do not use this product in patients with known sensitivity to any of the product’s ingredients. Dilution of the product prior to use is not recommended.
You may report a MuGard® adverse event to Soleva Pharma at 877-319-7272 or by emailing to
medinfo@solevapharma.com.
Please see accompanying full Prescribing Information.
References: 1. Peterson DE, Bensadoun R-J, Roila F; ESMO Guidelines Working Group. Management of oral and gastrointestinal mucositis: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22(suppl 6):vi78-vi84. 2. National Cancer Institute. Oral complications of chemotherapy and head/neck radiation (PDQ®): oral mucositis, health professional version. http://www.cancer.gov/cancertopics/pdq/supportivecare/oralcomplications/HealthProfessional/page5. Updated April 23, 2014. Accessed June 23, 2015. 3. Trotti A, Bellm LA, Epstein JB, et al. Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiother Oncol. 2003;66(3):253-262. 4. Fernandes LL, Torres SR, Garnica M, et al. Oral status of patients submitted to autologous hematopoietic stem cell transplantation. Support Care Cancer. 2014;22(1):15-21. 5. Nishimura N, Nakano K, Ueda K, et al. Prospective evaluation of incidence and severity of oral mucositis induced by conventional chemotherapy in solid tumors and malignant lymphomas. Support Care Cancer. 2012;20(9):2053-2059. 6. Nonzee NJ, Dandade NA, Patel U, et al. Evaluating the supportive care costs of severe radiochemotherapy-induced mucositis and pharyngitis: results from a Northwestern University Costs of Cancer Program pilot study with head and neck and nonsmall cell lung cancer patients who received care at a county hospital, a Veterans Administration hospital, or a comprehensive cancer care center. Cancer. 2008;113(6):1446-1452. 7. Afinitor [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2015. 8. Torisel [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc.; 2015. 9. Sternberg CN, Calabrò F, Bracarda S, et al. Safety and efficacy of sunitinib in patients from Italy with metastatic renal cell carcinoma: final results from an expanded-access trial. Oncology. 2015;88(5):273-280. 10. Herceptin [package insert]. South San Francisco, CA: Genentech, Inc; 2015. 11. Elting LS, Chang Y-C, Parelkar P, et al; on behalf of Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO). Risk of oral and gastrointestinal mucosal injury among patients receiving selected targeted agents: a meta-analysis. Support Care Cancer. 2013;21(11):3243-3254. 12. Elting LS, Keefe DM, Sonis ST, et al; Burden of Illness Head and Neck Writing Committee. Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer. 2008;113(10):2704-2713. 13. Mucositis Study Group of the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology. MASCC/ISOO Evidence-based Clinical Practice Guidelines for Mucositis Secondary to Cancer Therapy. http://www.mascc.org/assets/Guidelines-Tools/mascc%20isoo%20mucositis%20guidelines%20summary%207nov2014.pdf. Published November 7, 2014. Accessed June 23, 2015. 14. Epstein JB, Thariat J, Bensadoun R-J, et al. Oral complications of cancer and cancer therapy: from cancer treatment to survivorship. CA Cancer J Clin. 2012;62(6):400-422. 15. World Health Organization. WHO handbook for reporting results of cancer treatment. Geneva: World Health Organization; 1979. WHO offset publication 48.

